Clinical sample management requires laboratories to put a significant amount of thought and effort into record-keeping and reporting in order to meet compliance requirements.  Regardless of the type of compliance (HIPAA, Part 11, GLP, etc), managing clinical samples requires a commitment to actively maintain an audit ready-state. For many labs that are getting started with clinical sample management, this can be a daunting task. Check out the 3 tips below to get started.
Regardless of the type of compliance (HIPAA, Part 11, GLP, etc), managing clinical samples requires a commitment to actively maintain an audit ready-state. For many labs that are getting started with clinical sample management, this can be a daunting task. Check out the 3 tips below to get started.
Clinical Sample Management Compliance Tips
1. Traceability of Clinical Samples
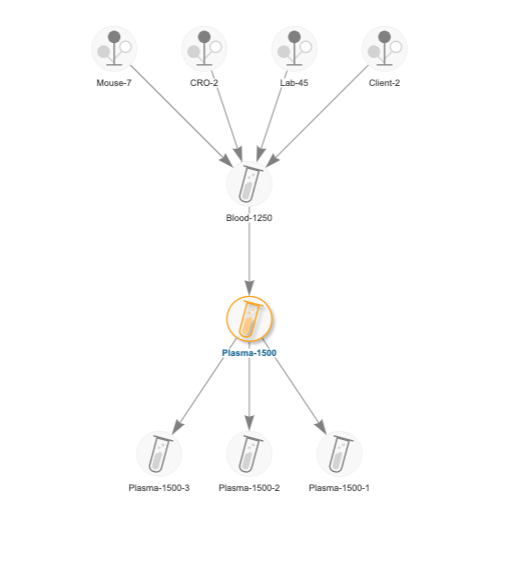
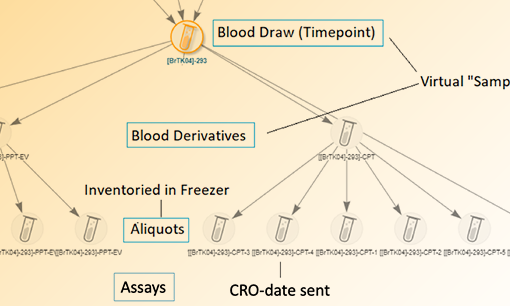
Your staff should be able to the “what, when, how, who” of every human-derived sample in the lab. Sample tracking should include the capture of all details around the collection, receipt, storage, processing and handling samples.
What? – Document the type(s) of samples you will receive/create including the descriptive metadata that you want to collect for each sample type.
When? – Capture dates for all events in the lifecycle of a sample including when it was collected, received, processed, stored, and shipped.
How? – Track step-by-step how tasks were performed in the lab in relation to a sample. Utilize sample management software to capture SOPs in workflows and audit trails.
Who? – Capture who in the lab performed any work or handling of the samples. This is both valuable for accountability and troubleshooting.
2. Sample Identification & Barcoding
Clinical samples should have clear and unique identifiers that are appropriate to the laboratory type. Among other considerations, do your samples need to be de-identified for a specific laboratory or can they have PHI in the identifier? Do your naming patterns easily provide information and context for a given sample?
Sample Receipt – Consider how samples will be labeled from the collection site and received into the laboratory. Do they need to be de-identified before use in the lab?
Laboratory-Specific Identification – Define naming patterns that account for the uniqueness of your samples, aliquots, derivatives, and repeat samples. Although you may also use barcoding, consider having your identifier be “human-readable” so that lab staff can identify samples on-the-fly.
3. Standard Operating Procedures
When managing clinical samples it is essential to have clear SOPs that document sample processing tasks and workflows. SOPs should accurately document the work to be done in the lab as well as capture any results from sample processing tasks.
Document – With the help of your team, draft an example of the sample lifecycle in the lab. Be sure to capture areas of possible deviation, key variables and decision points.
Create/Update SOPs – Compare the sample lifecycle with your SOPs to ensure procedures are aligned with what is taking place in the lab.
Evaluate – Conduct a “trial run” of your clinical sample intake, processing and storage to ensure SOPs accurately reflect real-world procedures.
Maintain – Define a schedule to regularly review processes and track any changes to SOPs using documented change requests. Internal audits should also be scheduled at set intervals to keep your lab in an “audit-ready” state.
4. Record Keeping
All work performed on clinical samples should be documented and include the same “what, when, how, who” details mentioned before. This should be a record of the actual work that was done, versus your SOPs, which layout a plan and methodology for the work. Keep regulatory requirements in mind when determining how this documentation will be captured, stored and shared.
Clinical Sample Management with LabKey Sample Manager
Efficient and compliant management of clinical samples requires sample management software that is both powerful and easy to use. Sample Manager is a cloud-based sample management system that helps:
- Track the full history and chain-of-custody for each sample.
- Register and definine samples using all relevant metadata
- Manage task-based workflows that accurately reflect SOPs
- Support barcoding and unique, human-readable sample ID creation
- Retain records of the complete life-cycle of each sample.
- Easily manage freezers and sample storage
Click here to learn about Sample Manager and take a product tour!