An ELN (electronic lab notebook) is an essential software tool for documenting experiments, reviewing compiled results, detailing meaningful conclusions, and collaborating with team members. As scientists work with increasing volumes of data, concerns over data reproducibility, data integrity, intellectual property and security/compliance have led to the wide ELN adoption in nearly every scientific discipline. Once viewed as little more than a paper-on-glass/word-processing application, ELN features have grown to meet the complexities of modern-day research. Below we outline some essential ELN features to look for when evaluating an electronic lab notebook for your organization.
An ELN (electronic lab notebook) is an essential software tool for documenting experiments, reviewing compiled results, detailing meaningful conclusions, and collaborating with team members. As scientists work with increasing volumes of data, concerns over data reproducibility, data integrity, intellectual property and security/compliance have led to the wide ELN adoption in nearly every scientific discipline. Once viewed as little more than a paper-on-glass/word-processing application, ELN features have grown to meet the complexities of modern-day research. Below we outline some essential ELN features to look for when evaluating an electronic lab notebook for your organization.
ELN Feature Areas to Consider:
1- Ease of use
The ELN you choose should be easy to use and implement. This is especially critical in driving adoption within the organization. That means the user interface should be intuitive for common functions. Ideally, your new ELN should not require extensive training to learn nor require sophisticated integrations with other software to be useful.
2- Templates
The ability to create and use templates of common notebook forms saves time by reducing duplicative work. The ability to quickly modify templated entry types is an essential feature for saving time and improving overall work satisfaction. By creating templates for specific scenarios such as purifications, cell passaging, or repeated analytical work, your team can save time and standardize the formatting of notebook entries.
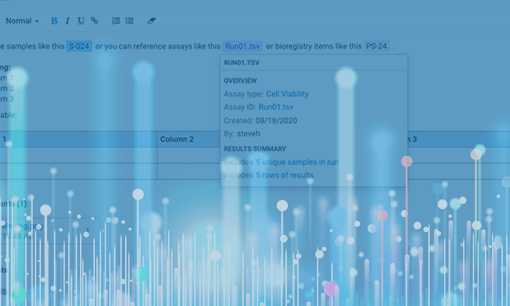
3- Data Links/Connection
Compiling data for meaningful write-up and conclusions should not require a huge investment of time and effort. By far the easiest way to surface data is for your ELN to have direct access to your underlying data management system that captures the data. This puts your relevant data at your fingertips while authoring and only external data will require effort to incorporate. Directly referencing data not only saves time, it also promotes data integrity and ensures that valuable data remains at the forefront of the decision-making process during the R&D lifecycle.
4- Attachments and Images
External files and images are important components of complex workflows. It should be easy to incorporate them into notebook entries in various formats such as pressure traces, spreadsheets, images, CoA’s or other QC documents, and SOP’s. The ability to bind these files with your signed notebook contents and view the attachments with a notebook entry can also be a huge time saver.
5- Collaborative Features
In the highly collaborative research environments of today, it is essential to have an ELN that makes it easy to work with other contributing scientists. The ELN should support collaborative authoring and a streamlined sign-off/review process for notebooks. It should be easy to find the notebooks that need your attention, comment or review. You should also receive reminder notifications for these actions when you aren’t in the application. These collaborative features facilitate important discussion and encourage timely and accurate documentation of work.
About the Biologics LIMS ELN
Biologics LIMS includes a full-featured electronic lab notebook seamlessly integrated with the Bioregistry, Assay Data and Workflow Management tools. This user-friendly ELN allows scientists to:
- Save time authoring and reviewing notebooks with templates, notifications and an intuitive interface
- Highlight valuable data by linking directly to bioregistry entities, samples and assays
- Collaboratively document and discuss notebook entries and referenced data
Click here to learn more about LabKey Biologics.