Biopharmaceuticals are increasingly being prescribed for a variety of diseases, from autoimmune disorders such as arthritis to neurological conditions like Alzheimer’s. Neutralizing antibody (NAb) assays are a critical component of biopharmaceutical development, helping inform researchers of potential product efficacy and patient safety. Having reproducible, repeatable NAb assay results will improve the product research pipeline and ultimately impact trial and patient outcomes. For this reason, efficient NAb data management is more important than ever to biomedical researchers.
With the advancement of plate and instrument technologies, NAb assays provide a high-throughput mechanism of evaluating the potential immunogenicity of the drugs they study. Teams must be able to set up plates accurately, produce consistent analyses, ensure appropriate quality controls, and keep track of data provenance in order to deliver NAb assay results that are reproducible, comparable, and reliable.¹ LabKey Server helps teams overcome core challenges in generating reliable NAb data in the following ways.
Facilitating Good Record Keeping Practices
LabKey Server helps scientists maintain good record keeping by:
- Directly importing instrument-derived results files and collating them into an analysis dashboard
- Improving data integrity by associating raw data files and results
- Providing a built-in graphical template designer that allows users to quickly create new plate layouts (supporting options for cross- or single-plate dilutions, and single- or multiple virus plates)
Streamlining NAb Data Analysis & QC
Improve the consistency and ease of NAb data management and analysis using LabKey Server by:
- Automatically calculating and generating neutralization curves and titers
- Removing ill-fitted and otherwise unsuitable data and maintaining those changes for future quality assurance
- Translating complex plate maps with dilutions and/or multiple viruses into the NAb dashboard so that results of each run may be viewed and graphed on a per-virus basis
Enabling Collaborative Analysis
LabKey Server can help researchers collaborate and share NAb data by:
- Centralizing raw file storage and analysis in a secure web-based interface
- Providing an interactive NAb Dashboard for collaborators to interrogate the data
- Integrating NAb data with other data types, presenting users with a comprehensive view
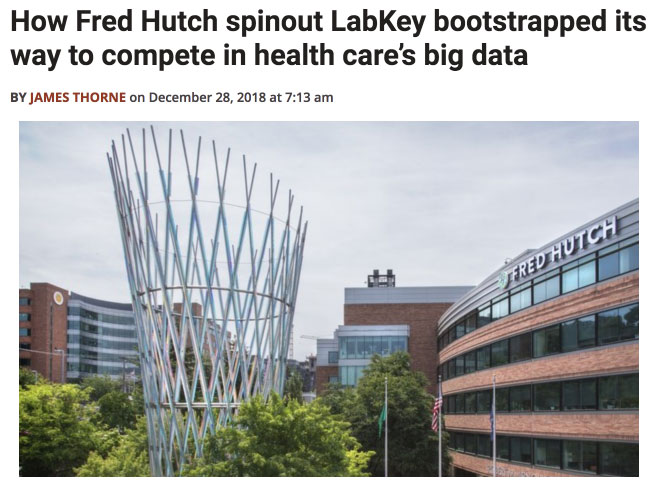
High-throughput 384-well NAb assays may contain hundreds of samples with dilutions across plates or within a single plate and the resulting graphs and views can be complex. The LabKey NAb Assay tools provide quick visual feedback allowing you to confirm a valid run or immediately correct and rerun if necessary. To learn more about using LabKey Server to manage NAb data, check out the NAb documentation library on the LabKey Support Portal or request a demo.
¹https://bmcimmunol.biomedcentral.com/articles/10.1186/1471-2172-12-33



 There are several ways in which the visibility into previously generated data provided by LabKey Biologics can prevent duplicate work in the lab. Perhaps a bench scientist is interested in understanding what protein expression occurs under a particular set of conditions. Unbeknownst to them, an experiment testing that same protein expression was completed 6 months earlier by another team member. Using LabKey Biologics, this researcher could search historical experiments with those same conditions and view their results, saving them the time and resources of conducting a new experiment.
There are several ways in which the visibility into previously generated data provided by LabKey Biologics can prevent duplicate work in the lab. Perhaps a bench scientist is interested in understanding what protein expression occurs under a particular set of conditions. Unbeknownst to them, an experiment testing that same protein expression was completed 6 months earlier by another team member. Using LabKey Biologics, this researcher could search historical experiments with those same conditions and view their results, saving them the time and resources of conducting a new experiment. A lack of visibility into your team’s previously generated data can also lead to duplicate records in your bioregistry. LabKey Biologics provides easy mechanism for searching/sorting existing data to locate previously registered entities, and also conducts an automated uniqueness check on each entity registered in the system. These tools ensure that related data is connected to the correct entity from the start, and removes the need for downstream data clean-up.
A lack of visibility into your team’s previously generated data can also lead to duplicate records in your bioregistry. LabKey Biologics provides easy mechanism for searching/sorting existing data to locate previously registered entities, and also conducts an automated uniqueness check on each entity registered in the system. These tools ensure that related data is connected to the correct entity from the start, and removes the need for downstream data clean-up.
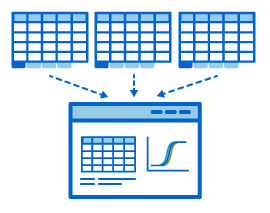 Providing support for multi-tabular excel output files and converting them into easy-to-read grids
Providing support for multi-tabular excel output files and converting them into easy-to-read grids LabKey Server’s Luminex data management tools help labs improve quality control of their data by:
LabKey Server’s Luminex data management tools help labs improve quality control of their data by: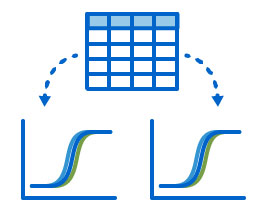 Logging changes to data records and allowing scientists to view the history of data transformations from the raw file to the analyzed results
Logging changes to data records and allowing scientists to view the history of data transformations from the raw file to the analyzed results
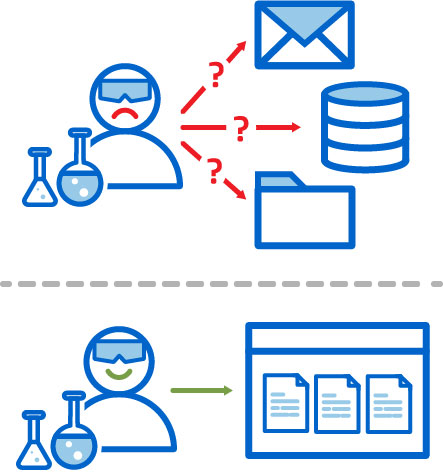
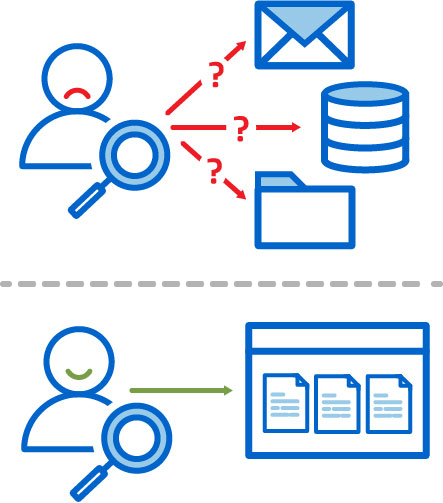 Team Members Know Where to Find Data
Team Members Know Where to Find Data


 Adam Rauch
Adam Rauch