A key function of LabKey Biologics is assisting with antibody development and registration. Registration allows all data from antibody production processes to be associated with the candidate. It also allows for the optimization of processes at each stage of antibody development by facilitating the comparison of antibody experiment data.
Registering Antibodies and Components
To register antibodies, LabKey Biologics provides an easy-to-use Bioregistry. Users can add an antibody description, aliases, and details about the molecule’s lineage. Then, users select the components of this molecule, generally composed of one or more protein sequences, and finalize the stoichiometry. The Bioregistry verifies that this entity, defined by its components and their stoichiometry, has not previously been registered.
Users can also register molecules in bulk through the user interface, or via API. This includes support for importing GenBank files.
Tracking Component Relationships
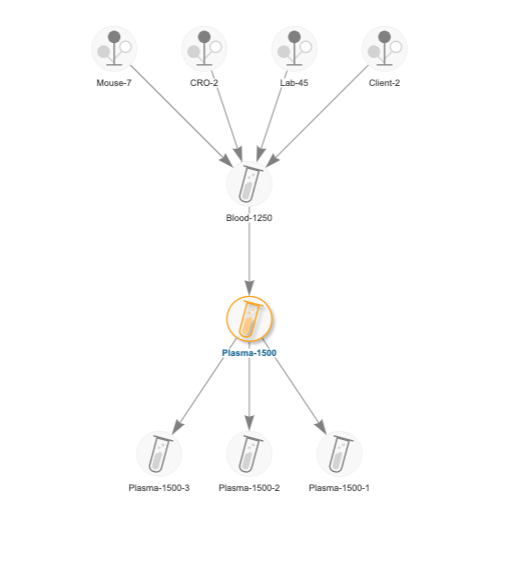
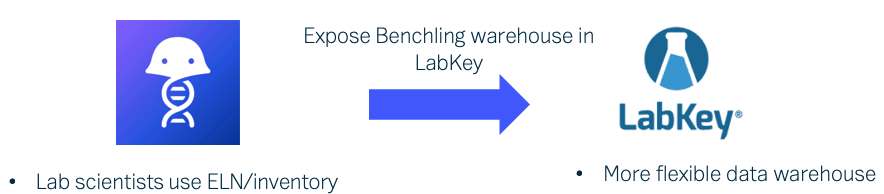
Antibody development typically utilizes an expression system to produce samples of a molecule and its variations. LabKey Biologics stores key physical characteristics about registered molecules and presents users with captured information about its components, with linkage to the expression system from which it is derived. The relationship between an expression system and its target molecule can be determined from the construct, media, and gene inserts used in the expression system. Users are able to import biologics assay data for samples derived from that expression system for comparison and deeper analytics. When a user registers a new expression system in LabKey Biologics, the application makes an explicit connection between it and the molecule of interest. This relationship is valuable for visualizing and navigating between the related expression system data and the molecule data.
Antibody Development Workflow
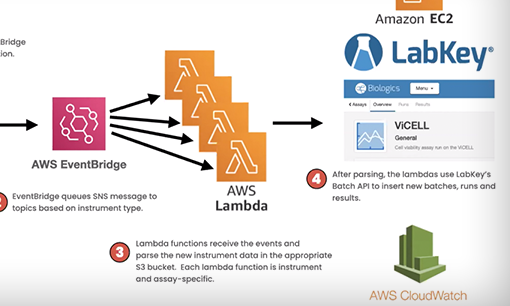
Proper coordination of the multifaceted activities involved with antibody development is essential to ensure high quality results. Research managers must orchestrate all activities in their lab to facilitate communication, prevent bottlenecks, and prevent data from becoming siloed. LabKey Biologics provides a centralized system for managing ongoing research workflows and storing data generated across team members. This includes features to generate configurable work requests and track laboratory tasks. By maintaining the relationship between tasks and their resulting data LabKey Biologics ensures smooth handoffs and provides researchers with a holistic view of research operations and workflow progress. For example, a user can navigate from assay result data to the original request for it, facilitating operational visibility.
Additional Resources
LabKey biologics has features to assist researchers in nearly every stage of the biotherapeutic development pipeline. To learn more about LabKey Biologics, please explore the links below.
> Bioregistry
> Biologics Workflow Manager
> Biologics Assay Management
> Electronic Lab Notebook