Tag: Sample Manager
Preparing for a Lab Audit
For researchers working in a regulated laboratory environment, preparing for an audit can be a daunting task. This is especially true if your records, staff training and SOPs have been neglected for a long period of time. Trying to play “catch up” once you learn about an impending audit will almost always be a losing proposition. Once you learn about an upcoming audit it will help to first understand the scope and depth of the audit. This will help you prepare your materials and allocate time and resources. However, the best way to prepare for a lab audit is to always be ready for an audit.
Tips for the “audit-ready” laboratory:
- Make sure your Standard Operating Procedures are up-to-date.
In order to understand how your lab is processing samples or collecting data, the auditor will be looking for clear SOPs that accurately describe the work in the lab. In general, once SOPs are created, they should be reviewed each year by people doing the work – and edited and versioned appropriately. Keeping your SOPs accessible and accurate will make preparing for an audit less stressful. If you have created corresponding lab workflows, you should also make sure that these accurately reflect your documented procedures. - Ensure staff training records are up-to-date, complete and easily accessible. Auditors will check that the staff performing work in the lab are competently trained in the work they are performing. Keep accurate records of staff training and make sure that staff are getting regular refreshers as lab processes are updated.
- Have audit-ready sample records.
This can be challenging if your lab doesn’t follow standard procedures for record-keeping, or if your sample management software isn’t already doing this for you. If you’re not sure if your sample management system keeps audit-ready records, start by asking yourself- “If I picked a sample at random, would I be able to identify everything that happened to this sample?” Follow the “Who/What/When” approach – who performed that on what sample when? Sometimes the answer may be yes, but it could be a jumble of assorted records spread between notebooks and electronic systems. If you can’t establish the chain of custody easily and quickly, it may be time to reconsider your sample management system.
LabKey Sample Manager makes complete sample records and chain-of-custody easy to access with our Timeline feature. Timeline shows the complete chain of events for an individual sample from initial registration, to metadata/status updates, all storage events, related assay data and addition of samples to a workflow job.
Creating a Laboratory Workflow for Sample Management
A laboratory workflow is a set of procedural rules used to manage and coordinate tasks between people and systems in the lab. Lab workflows for sample management ensure that all steps and requirements in a defined process are correctly  followed to reduce the time and cost of sample handling, preparation and data collection while ensuring the quality of the sample and any associated data is maintained. Laboratory workflows are often guided by standard operating procedures (SOP), and are most effective when thoughtfully developed to reflect real-world practices in the lab. Sample management software or other lab management software is often used to help define and manage workflows.
followed to reduce the time and cost of sample handling, preparation and data collection while ensuring the quality of the sample and any associated data is maintained. Laboratory workflows are often guided by standard operating procedures (SOP), and are most effective when thoughtfully developed to reflect real-world practices in the lab. Sample management software or other lab management software is often used to help define and manage workflows.
See how Sample Manager can help manage lab workflows. Learn More
Generally, laboratory workflows should encourage best practices that:
-
- Improve adherence to quality standards
- Promote compliance to regulatory requirements
- Maximize laboratory throughput
- Efficiently allocate laboratory staff and resources
Given the limited resources of most labs, how can a lab manager develop and implement workflows that encourage these overarching goals? Below are a few tips to get started
Start small, but be impactful with the laboratory workflow you choose.
When starting to develop laboratory workflows, it is tempting to try and create your entire workflow all at once on a macro level. Not only could that approach be overwhelming, it also skips important workflow analysis steps, and could lead to missing sub processes or important tasks within the lab. Instead, start by choosing a specific process that has the most impact for staff and the output of your lab. This may be a particular work area, or common set of procedures. For example, rather than focusing on the entire lifecycle of a sample within the lab, it may be better to start by focusing the workflow for receiving samples. Keep in mind that this workflow may have several subprocess/workflows such as those for sample labeling, quality control checks, verification of manifest information, or freezer management.
Identify obstacles and constraints within your lab workflow.
As you detail the process from which you create your lab workflow, make sure you identify any bottlenecks, obstacles or constraints in the process as well as outlining the solution for those issues. When thinking about tasks in a sample processing workflow be sure to consider:
-
- What are the important measurements and data points to track during the process? For example, is it important to capture the lot number of the reagents used, the temperature inside the building, the minutes a tube has sat in a reagent? By assessing what is the most important information to track, you will avoid capturing unnecessary information and cluttering your sample management software.
- Are there tasks that should be repeated if a specific measurement is not within a specified range? How are those points assessed and tracked? By noting these measurements and stopping points in the process, your workflow will be more detailed and help ensure the quality of downstream data.
- Do certain tasks need to be performed quickly? By knowing how time sensitive certain aspects of the workflow are, you can then better assess capacity and demand for staff and resources.
Keep capacity and allocation of laboratory staff, resources and equipment at the front of mind.
This is important for both resource planning in the laboratory, and also for understanding the limitations of the workflow. If you only have one tissue culture hood, and it’s an integral part of your workflow, you’ll need to take this into account and set accurate expectations. Proper allocation of resources can also lead to an improvement of productivity and reduction of operating costs. A few considerations include:
-
- Workstations: Are they properly set up to handle the required work? Does their physical location align to your workflow or create unnecessary bottlenecks.
- Scheduling: How does the availability of resources align with the amount of work to be performed and staff scheduling? How can you reduce interruptions from equipment calibrations and maintenance?
- Workstations: Are they properly set up to handle the required work? Does their physical location align to your workflow or create unnecessary bottlenecks.
The more detailed your process is, the more complex your lab workflow is likely to be. However, it is best to think about your lab workflow efforts as an ongoing journey of optimization rather than finite destination. As a final word of advice- be sure to include your staff in the workflow development process. This will help you get buy-in from stakeholders and give you valuable insight for establishing the optimal lab workflows.
Want to learn more about our laboratory workflow solutions?
>LabKey Sample Manager – product overview and tour
>Request a Demo
Should I Use Free Sample Management Software in My Lab?
 For many laboratories, try to adopt free sample management software can be tempting due to the perceived cost savings. Although free sample management software may appear to be a viable option, many of these systems lack the features, flexibility, customization and support that labs need. In the long run, free sample management software can prove to be quite costly due to wasted time and resources in trying to implement a system that is poorly designed and ill-suited for even the most basic needs of many labs.
For many laboratories, try to adopt free sample management software can be tempting due to the perceived cost savings. Although free sample management software may appear to be a viable option, many of these systems lack the features, flexibility, customization and support that labs need. In the long run, free sample management software can prove to be quite costly due to wasted time and resources in trying to implement a system that is poorly designed and ill-suited for even the most basic needs of many labs.
Free sample management software is often missing:
- Complete and auditable record keeping.
Most free systems have a severe lack of audit capabilities. Some will even ask you to upgrade to a paid version for what is still limited audit functionality. Your sample management software should capture a full audit trail for each sample. This will allow you to track the full chain of custody, meet regulatory requirements, and have confidence in the data stored in your system. - Flexibility, customization and data integrity.
Free sample management tools often lack the needed data validation and enforcement capabilities of robust sample management software. This can impact the integrity of data in the system and lead to costly errors. Free sample management systems will also often limit options of flexibility and customization. This includes forcing limits on user-defined fields or forcing the lab to change its processes to fit the system. Your sample management software should have the ability to be molded to your lab processes and requirements, not the other way around. - Options for scalability and growth.
Often, free sample management software will limit the number of projects, data rows or users in the system. This isn’t usually an issue until a laboratory reaches these limits and is surprised by the cost to upgrade. Using a sample management system that is designed to grow with you- both in data size and functionality, will keep your lab running smoothly in times of growth and change. - Maintenance and support.
Free sample management systems aren’t typically set up to provide support to their clients or solve their problems. Support and maintenance costs time and money that a free system is simply not able to expend. Underestimating the time it takes to maintain a system, get advice from a live person when you need it, and addressing bugs can mean more time away from your science and the critical work of your lab.
LabKey Sample Manager is user-friendly & powerful- and it won’t cost a fortune.
LabKey Sample Manager is an affordable and easy-to-use sample management software designed to help labs efficiently track samples, define laboratory workflows, and unify samples with assay data. Features include:
Sample Tracking – End-to-end sample tracking features including chain-of-custody tracking, sample types and sources, and lineage views
Freezer Management – Flexible and intuitive management of freezer capacity and sample storage locations.
Sample Data Integration – Integrate assay data with your samples and assign metadata for a complete picture of your ongoing experiments
Lab Workflow Manager – Assign samples to user-defined workflows and monitor the workflow completion status at each stage for every sample
Take a product tour or request a demo today!
Enhancing Lab Efficiency: Strategies for Clinical Sample Inventory Management
What’s Possible With Lab Sample Barcoding?
What is Sample Tracking?
 Sample tracking is knowing the “what, where and why” of your lab samples.
Sample tracking is knowing the “what, where and why” of your lab samples.
What is sample tracking? Let’s start with a common scenario. A post-doc from two years ago collected a few dozen samples that would be really valuable to re-analyze with your new lab toy. But where are those samples? You could consider trying to regenerate the samples rather than finding them in your freezer. But then you realize you don’t have that strain of mice anymore, so you go searching.
First, you go to their old notebooks and see if you can track them down. After searching in several notebooks, you find that they stored them in the -80C in a box called “My samples”. So, you look through your freezers to finally find the box, and see that they are labelled with a date (yay!) and a numeric value 1-40. You go back to the lab notebook (which one was it again?!) and map these numeric values to the information stored in the notebook.
The scenario above is a concise example of how the right sample tracking procedures and sample management software could save time and resources.
Click here to learn how Sample Manager can help your lab.
Sample Tracking in the Lab
Accurate and efficient sample tracking is an important function of all well-organized labs. In order to identify, use and understand the context of a sample after it’s been collected, lab staff should at a minimum collect and notate the following information:
- the properties of the sample (name, unique ID, where it was derived from, volume, etc.)
- the location of the sample
- any activities or assays that the sample has been a part of
- the current status of a sample at any given time
Many labs are now capturing this information electronically, whether it’s via a spreadsheet or with a sample management system. Thoughtfully designed systems and procedures are key to making sample tracking an easy and efficient process, however, designating resources upfront for these efforts can be difficult to prioritize for some labs.
Tracking Samples with LabKey Sample Manager
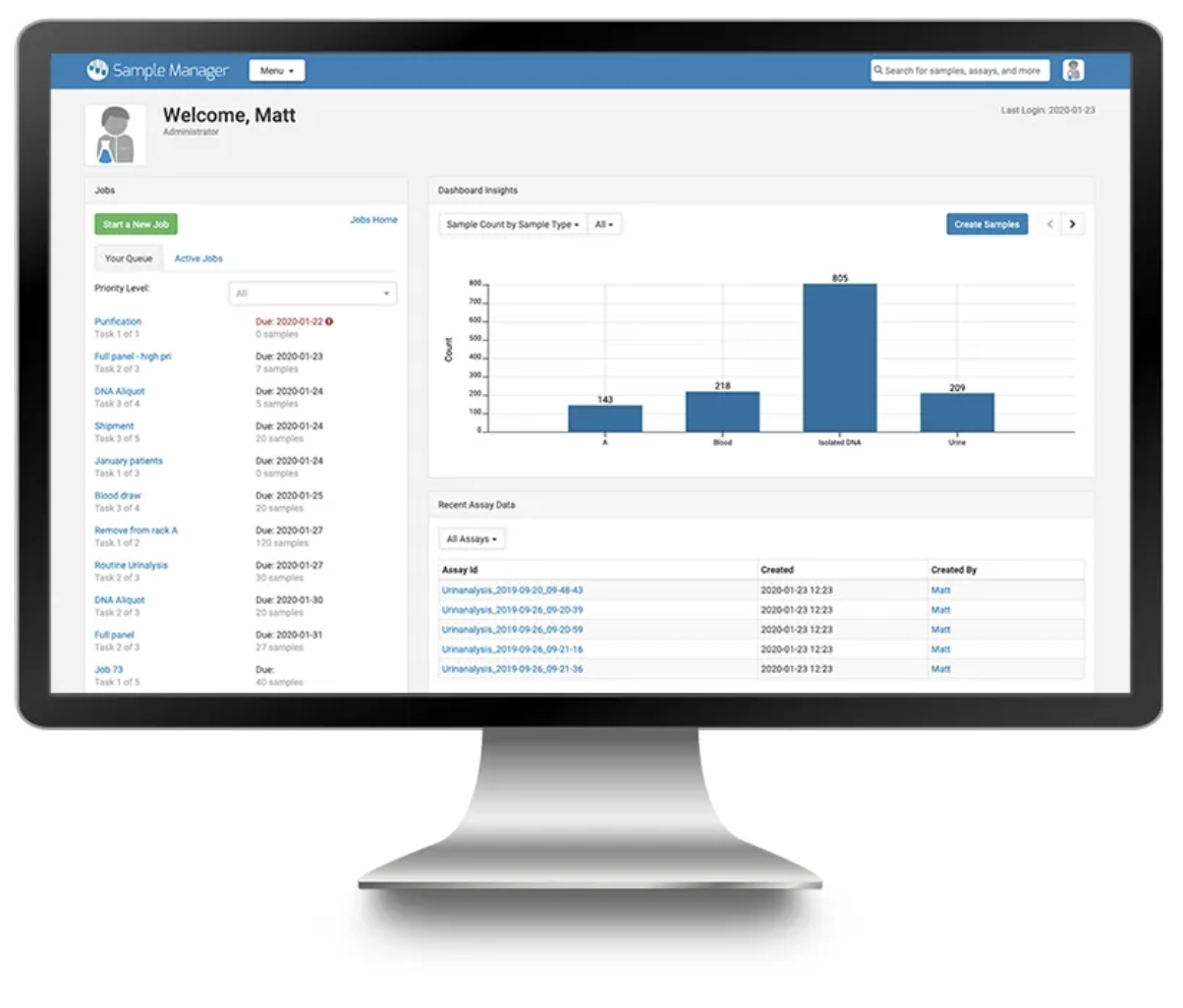 LabKey Sample Manager is an intuitively designed sample management application that brings ease and efficiency to sample tracking. The application has various features to assist in sample tracking including:
LabKey Sample Manager is an intuitively designed sample management application that brings ease and efficiency to sample tracking. The application has various features to assist in sample tracking including:
Sample Timeline – Track chain-of-custody and events for a samples such as assays performed, volume changes, and inclusion in workflow jobs.
Sample Sources – Assign sources to a sample to better understand the broader picture of the sample within the laboratory, experiment or study.
Sample Types – Organize samples by type and add data fields for that type to describe attributes of samples for easy tracking.
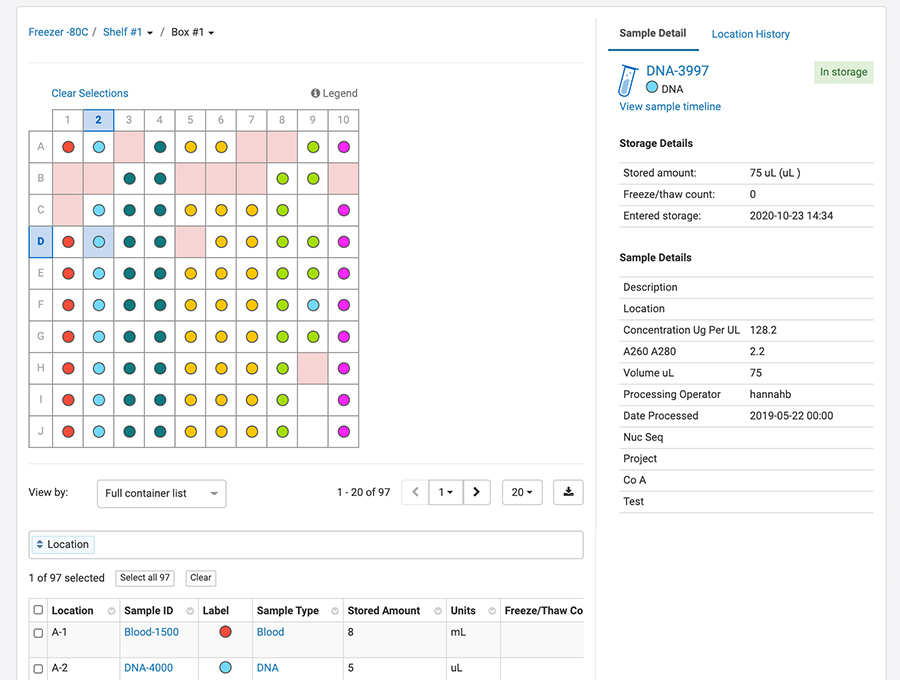
Freezer Management – Track the storage location of samples and freezer capacity.
Unique Sample IDs and Naming Patterns – Each sample in the system is required to have a unique name/id within its sample type. You can either choose the unique ID yourself or have it generated by the system using a naming pattern that can be customized by an administrator.
Tools for Tracking Sample Lineage – Sample Manager includes a lineage graph that provides a visual representation of the parentage of samples as well a lineage grid can be helpful when viewing lengthy lineages or derivation histories.
Click here to take a tour of Sample Manager!
Cool and Collected: Essentials of Lab Freezer Organization
Three Reasons Freezer Management Software is Vital
If you’ve worked in a lab, you’ve likely used some form of freezer management to manage your sample storage. For some labs, this may simply be a paper lab notebook where freezers, boxes, and sample locations are annotated. Others may periodically update an Excel file with samples stored in the freezer and place a print out of the spreadsheet on the freezer itself. However, for truly efficient and organized labs, nothing beats having freezer management software to manage your freezer capacity and sample inventory. Below are 3 key reasons why you need software to manage your lab freezer storage.
1. Your samples are precious and should be treated that way.
Samples can be one of the most precious things in the lab, sometimes they are even irreplaceable. The time spent collecting, processing, and collecting data from samples means they are valuable not only to your research but also to the cost efficiency of your lab. Misplacing and looking for lost samples can be a huge waste of time for laboratory staff and even more so if the samples are never found. Using freezer management software ensures that your freezers are kept organized and reduces the chances that you will misplace valuable samples.
2. Excel and paper-based freezer management can’t keep up with busy labs.
Although Excel and paper-based systems may be able to keep up with the needs of a very small lab, they are very difficult to keep updated with accurate information and definitely don’t meet the needs of larger or growing labs. Busy staff in the lab will need to manually/visually judge freezer capacity, update multiple sheets and samples when making simple changes, and have to manually keep track of sample volumes and freeze/thaw counts. This method of freezer management is highly prone to errors from day-to-day use not to mention during scheduled clean-outs and organization of the freezer.
3. Complete and accurate auditing is nearly impossible without freezer management software.
Auditing and organizing your samples is a much easier task with freezer management software. You’ll have a full record of all check-in/check-out events for samples, by which staff, and a full history of the location and movement of samples in the freezer. This level of completeness and accuracy is much more difficult to achieve using paper or excel. Freezer management software captures all of the information you need for a comprehensive audit of your freezer storage and samples.
Freezer Management with LabKey Sample Manager
With Sample Manager, we have introduced a sample-centric freezer management solution into the application. This means you can create samples, add metadata and lineage information, perform work and even upload assay data — all without having to use the freezer management tool. Of course, if you plan to store your samples, freezer management is there to help by:
- Managing your freezer with an end-to-end sample tracking system that stores the entire sample history and chain of custody in one place
- Providing a customizable, flexible structure to accurately reflects the physical freezers in your lab
- Tracking samples in and out of the freezer (freeze/thaw counts) and automatically calculating and tracking sample volumes
- Helping you to easily find samples in the freezer and move samples between freezers
- Tracking and maintaining full audit logs of the freezer and of the samples
The best way to encourage users to adopt a new system is by providing them with benefits that actually help them- save them time, make tasks simpler, etc. Additionally, when a system becomes the primary source of truth for team members, it becomes easier to adopt because that’s where the information lies. Click here to learn more about our freezer management solution.
Upgrade Your Freezer Management System Without the Pain
Migrating data from a freezer management system can be hard. So difficult in fact, that many labs continue to use an outdated system long after it is no longer serving their needs. Sometimes, the legacy system has even become a hindrance to sample management operations. However, this shouldn’t deter you from taking on the challenge of upgrading your freezer management software and reaping the rewards of improved organization and management of your lab samples. Below are a few tips for a painless switch to a new freezer management system.
 Plan and prepare for your new freezer management system.
Plan and prepare for your new freezer management system.
Develop a plan for migrating to your new system that includes a timetable for which freezer data will be migrated, and roles/responsibilities during the process. Make that your team is trained on the new system and that you are communicating clearly throughout the process.
Backup your legacy data.
Before anything, it’s important to have a backup of data for record-keeping and disaster recovery. During the migration process, this backup will serve as a record of your legacy system data- some of which may not transfer over to the new freezer management system. Create system-wide backups for recovery, but also human-readable backups for data mining if an unlikely event happens.
Determine what will be migrated.
Take this as an opportunity to clean up legacy data and items in the freezer. This might even be a good time to defrost and clean your freezers if they are not on a regular defrosting schedule. Focus on the essential samples and related data to keep your system and your freezers clutter-free. For each sample type, determine the list of fields exported from the legacy system and how they will map to the new system. Document data types, field validation (required fields or a specific range of values), and any other requirements such as relationships between samples and sources.
Utilize your resources – ask for help.
Many vendors offering freezer management system offer migration services, so ask for help! If the system you’re moving to doesn’t offer this, consider hiring a consultant or contractor to help if your team members are too busy or you need advice. When choosing a system, make sure to consider the support you will need to implement your new system.
LabKey Sample Manager provides:
- Easy, straightforward data import format to register your samples and their locations in a single step
- Unlimited user-defined fields allow teams to capture any data necessary
- Migration services and customer support when needed
Click Here to learn more and take a tour!